Bad metals gone good: the key to sustainable energy transportation
16 November 2022
Cardiff University scientists have linked with their Brazilian counterparts in October of 2022, across both Cardiff and Cuiabá, to turn bad metals to good in the world of energy transportation.
In an international effort, scientists from The Federal University of Mato Grosso (Brazil) and Cardiff University joined forces over this important and forward-thinking field of research.
Their effort is all to show how using a combination of advanced calculations and a knowledge of chemical and physical properties can turn the crystal structure of ‘bad’ metals into ‘good’, so that they may become high-temperature superconductors.
When we talk of ‘bad’ metals, we are referring to the fact they function as inefficient conductors under more normal conditions.
We need ‘good’ metals therefore to ensure future green, sustainable practices in Chemistry, and specifically in energy transmission.
Today’s continual search for sustainable and renewable energy-generation strategies, often faces the challenge of distributing energy from remote power plants to consumers. This method is less than ideal because transporting electricity along conventional metal wires involves substantial heat losses due to the electrical resistance of the wires.
The beauty of superconductors is that they have zero electrical resistance. Which of course then means that they generate zero heat when used to transport energy and are therefore highly efficient and sustainable.
So, the benefit of the high temperature superconductor is that they promise transmission without the worry of losses, even over long distances.
However, it is known that materials may only become superconducting at very low temperatures, and most metals must be cooled to near absolute zero ( -273.15 °C ) for the superconducting qualities to emerge.
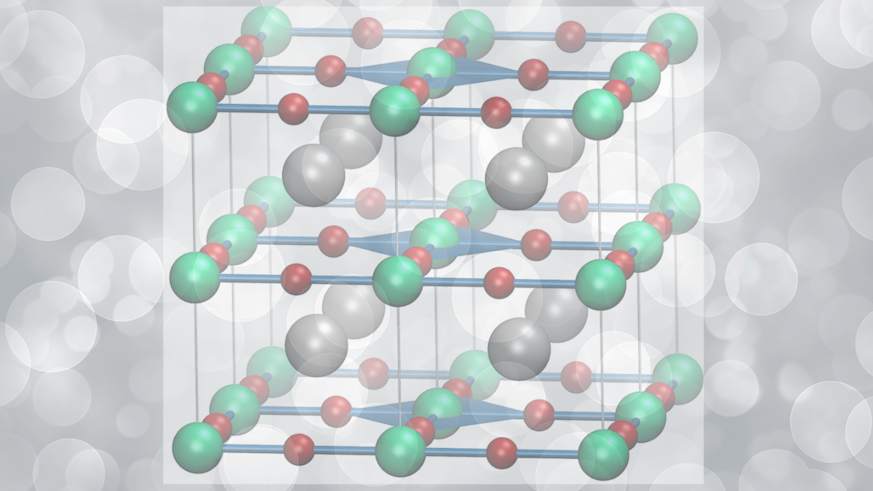
The highest temperature superconductors currently know are based around copper oxide (CuO2). By adding other metals to the mix these materials known as cuprates have pushed the temperature for superconductivity up to -135 °C. This still requires significant refrigeration and so they only find specialist applications and cannot be used for power transmission.
Even though these cuprate based materials are superconducting at low temperatures they are inefficient conductors under more normal conditions and so can be thought of as “bad” metals.
The original theory of how superconductivity comes about was put forward by Bardeen-Cooper and Schrieffer in 1957, and more recently, the substitution of copper by nickel in corresponding oxide compounds has opened a new area of research, in which chemistry is playing the key role in developing the right process for the synthesis of these ‘strange’, ‘bad’ metals.
A key step consists of tuning the synthesis to yield a specific number of electrons per atom of nickel similar to the copper oxides. Of importance for superconductivity is the need to confine electrons in narrow spaces, close enough to form Cooper pairs, but not too close that they risk reducing conduction.
In this work, advanced calculations are used to investigate how the interplay between electron kinetic energy and local electron-electron interactions drives bad metallicity into an unconventional superconducting state, and how chemistry can use this knowledge for better, sustainable high-temperature superconductors.
L. Craco, A. S. de Arruda, S. Leoni, Emergent normal-state Mottness in the infinite-layer NdNiO2 superconductor, Phys Rev Research 4, 043036 (2022).